Abstract
Background
In spite of the improvements in clinical care of children with Sickle Cell Disease (SCD), painful vaso-occlusive (VOC) crises, recurrent admissions and long hospital stays contribute to the disruption of the social and school life of children and adolescents with SCD causing a poor QoL.
Limited information is available regarding QoL of children, adolescents and young adults with SCD and their caregivers during standard of care and after bone marrow transplantation in Italy even though Italian patients participating in international meetings or global surveys highlighted the importance to improve QoL (Strunk C. BMC Proc. 2020, Osunkwo I. Am J Hematol. 2021). Moreover, no mention is given to QoL in the current AIEOP Recommendations for the Management of Children with SCD in Italy. The availability of new treatment options for SCD highlights the need to improve QoL evaluation before and after treatments.
Our Center decided, therefore, to include QoL evaluation as part of comprehensive care for patients with SCD. This study has the following aims: to describe the QoL of children, adolescents and young adults with SCD undergoing standard care or after disease curative treatments (bone marrow transplantation) and the QoL of their caregivers; to evaluate the correlation of QoL with clinical-haematological and therapeutic variables.
Methods
Health Related QoL was examined with the Patient-Reported Outcomes Measurement Information System (PROMIS) questionnaires in Italian: Parent Proxy Profile-49 v2.0, Pediatric Profile-49 v2.0 and 57 Profile v2.1, exploring 8 domains: Pain, Fatigue, Anxiety, Depression, Physical Abilities, Peer Relations, Sleep Disorders, Pain Interference. An English version was available for English speaking parents.
Patients and caregivers accessing the SCD Clinic starting May 2021 were given the paper version of the questionnaires; due to the COVID pandemic and the limited access to the SCD Outpatient Clinic, a link to a Google online version of the questionnaires was provided to all teenagers and young adults, through their mobile devices. .
PROMIS Scores were standardized through the Health Measures Scoring Service (healthmeasures.net). For the descriptive analysis, the T-score was obtained, for each patient and for each PROMIS domain (symptom or function), classifying impairment in each domain as normal, mild, moderate, or severe. The Student T Test for comparisons of the means among samples and the Wilcoxon Test for the sum of ranks were used in the statistical analysis of normal and non-normal continuous variables. For the correlation analysis between continuous variables of which at least one is not normal, the Spearman Correlation Test was used. The values with p<0.05 were considered statistically significant.
Results
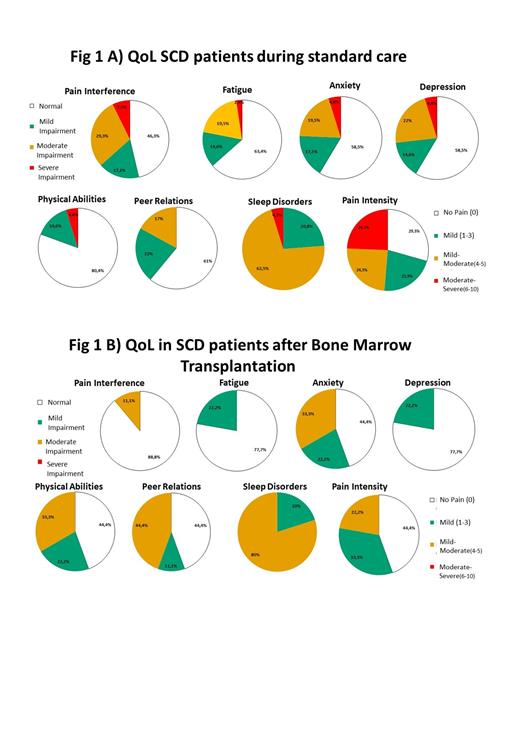
All patients and parents approached accepted to perform the questionnaire. The study involved 18 caregivers and 50 patients (25% F, mean age 16.4 years, 74% HbSS, 76% from Africa): 41 undergoing standard care (7 no therapy, 34 Hydroxyurea or chronic transfusion) and 9 who received HSCT. 37 patients (74%) and 8 parents (44%) completed the online Google version of the questionnaire. The standard of care patients displayed mild to severe symptoms in various domains (Figure 1A); in the transplant population there was impairment in QoL, with less severe impairment in most of the domains, especially in the pain domains, than what was in the standard of care group. (Figure 1B).
Anxiety levels and depressive symptoms were greatest between the ages of 14-26, compared to younger ages (p 0.018).
Parents do not have the same perception of the disease as their children: they appeared to overestimate the domain of pain and fatigue and underestimate anxiety and depression (p <0.001). Sleep quality was impaired in both affected and HSCT patients.
The number of hospital admissions in the previous year correlated with worse QoL (p 0.04), while the number of painful VOC showed a tendency towards significance (p 0.07); there was no difference with the other domains. Updated results will be presented.
Conclusions
Our data show the feasibility of evaluating QoL during routine visits and also remotely. Impairment of QoL is already present in a subgroup of young patients. Even after HSCT, QoL is not optimal but personal, social, and economic reasons need to be taken into account to adequately interpret the results. Longitudinal assessment to look at QoL will be important.
Biffi: BlueBirdBio: Consultancy, Other: Advisory Board. Colombatti: Global Blood Therapeutics: Consultancy; BlueBirdBio: Consultancy; NovoNordisk: Consultancy; Novartis: Consultancy; Forma Therapeutics: Consultancy; Addmedica: Consultancy; Global Blood Therapeutics: Research Funding; BlueBirdBio: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal